A Potential Lead Molecule for Treatment of Estrogen-Positive Breast Cancer
A potential anti-cancer lead molecule
Breast cancer (BC) is the second leading cause of death amongst women (1.7 million new cases diagnosed in 2012), where estrogen receptor (ER)positive BC accounts for 75% of cases. Approved drugs include selective ER modulators (tamoxifen antagonizes the binding of estrogen to ER and aromatase inhibitors (AIs) directly inhibit the production of estrogen). The adverse side effects of these drugs accentuate the need for new drugs that can provide enhanced efficacy, reduced toxicity, reduced drug resistance and longterm cancerfree survival.
Technology Summary
We present acetyl plumbagin (AP), a molecule that selectively inhibits ER-positive BC cell growth in vitro as well as in vivo and induces apoptotic cell death by disrupting cholesterol mechanisms, while showing negligible hepatotoxicity and general toxicity in mice.
How It Works
 Cholesterol has a vital role in the synthesis of new cell membranes and formation of lipid rafts in which receptors are embedded. Because cancer cells divide more rapidly and overexpress membrane receptors compared to normal cells, they have greater demand for cholesterol in order to sustain proliferation and cell membrane integrity. Cholesterol is also used for the biosynthesis of estrogen which further stimulates growth and proliferation of ERpositive BC. Cholesterol lowering drugs such as statins have been shown to reduce cancer growth in vivo. AP acts as a cholesterol modulator as it disrupts lipid rafts and depletes cholesterol from ERpositive BC cells, resulting in tumor growth inhibition and activation of apoptosis (Fig. A). AP reduces cell viability, disrupts mitochondrial function, activates caspases9 and 7 resulting in PARP1 cleavage, DNA damage and apoptosis.
Cholesterol has a vital role in the synthesis of new cell membranes and formation of lipid rafts in which receptors are embedded. Because cancer cells divide more rapidly and overexpress membrane receptors compared to normal cells, they have greater demand for cholesterol in order to sustain proliferation and cell membrane integrity. Cholesterol is also used for the biosynthesis of estrogen which further stimulates growth and proliferation of ERpositive BC. Cholesterol lowering drugs such as statins have been shown to reduce cancer growth in vivo. AP acts as a cholesterol modulator as it disrupts lipid rafts and depletes cholesterol from ERpositive BC cells, resulting in tumor growth inhibition and activation of apoptosis (Fig. A). AP reduces cell viability, disrupts mitochondrial function, activates caspases9 and 7 resulting in PARP1 cleavage, DNA damage and apoptosis.
Why It Is Better
The fractal design and single layer approach of the KAUST technology offers advantages over the standard MEMS parallel plate design and over CMOS fractals. Because of the configuration of the conductive plate in previously reported designs, unwanted parasitic or unintentional capacitance is generated. The KAUST innovation suppresses this parasitic capacitance because the signal and ground terminals are on the same plane and there are no top and bottom plates. Resonant frequency and Qfactor increase simultaneously. In addition, the geometry also almost completely eliminates the problematic plate warping that can be destructive to the functionality of any MEMS device.
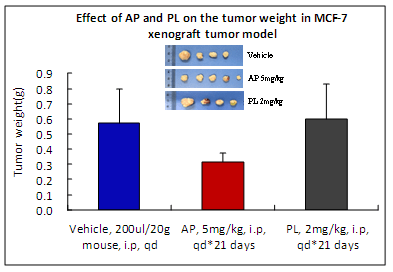
AP inhibited in vivo tumor growth up to 45% at 5mg/kg dose as compared to PL at 2mg/kg dose. A dose of 5mg/kg of PL was extremely toxic to animals.
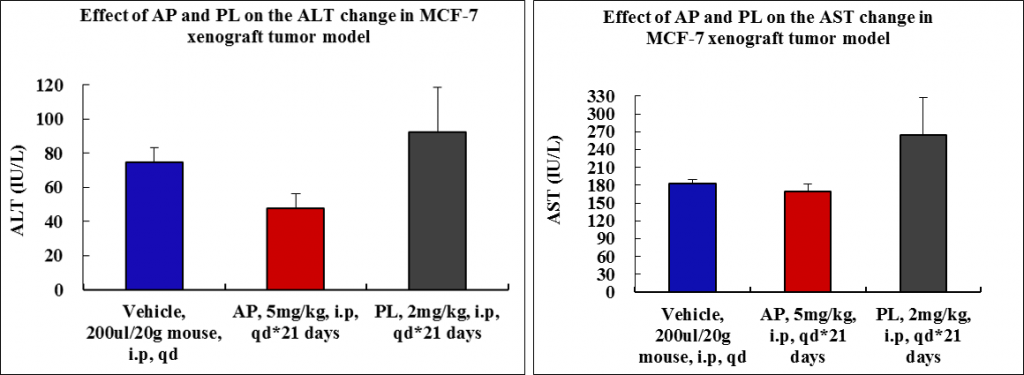
Toxicity of AP is minimal as compared to PL (no animal died; minimum body weight loss as well as low alanine aminotransferase and aspartate aminotransferase values).
AP acts by depleting cholesterol and inducing apoptosis and has broader application potential as compared to currently used tamoxifen and AIs.
IP Protection
KAUST has several patents pending for this technology.
Invention Track Code
2012-008

Benefits
- Anti-cancer activity: Induces apoptosis in cancer cells and inhibits tumor growth.
- No in vivo toxicity: Molecule causes no liver damage or impairment of liver function.
- Selective action: Targets ER-positive breast cancer cells and not normal cells.
Applications
- Cancer treatment for ER-positive breast cancer.
- Cholesterol modulation: Has the potential to be used as a cholesterol modulator in
addition to its use for cancer treatment. - Mobile handheld devicesAdjuvant therapy: Potential adjuvant drug to reduce the induced toxicity of
chemotherapeutic drugs.
