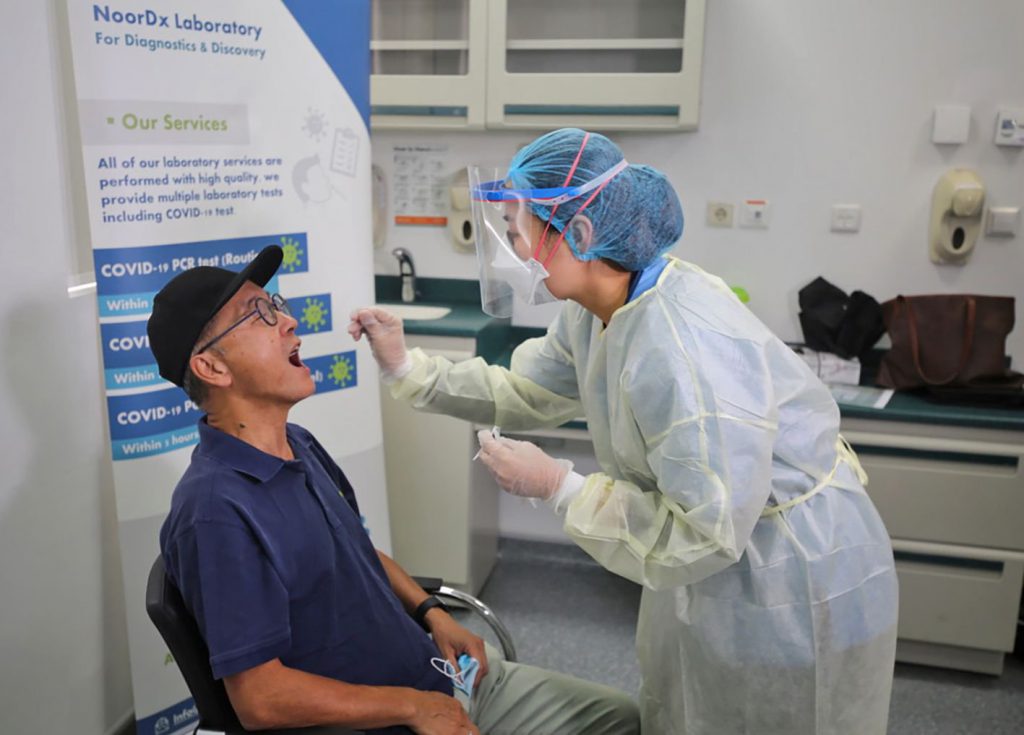
A KAUST-developed COVID-19 RT-PCR test, the Kingdom’s first, is now SFDA-approved. KAUST startup Noor DX launched the service at KAUST Health last week. Located in the Core Labs at KAUST, Noor DX provides a full suite of processing equipment using biotech diagnostics developed by Professor of Bioscience Dr. Samir Hamdan and his Rapid Research Response team. KAUST President Chan was the first to be tested.
Since the onset of the pandemic, the number of daily PCR tests in the Kingdom has risen from a few thousand to between 75,000 and 110,000 a day. Saudi Arabia has leveraged kits developed outside the country to meet demand. In-house capability increases self-reliance while reducing the wait and related costs. The result is an economical single-step, multi-use RT-PCR test priced significantly lower than previous tests.
The service begins at KAUST, and will expand to include communities in-Kingdom. People can expect high-accuracy results delivered to their personal devices via QR code technology within 24-hours, with most results returned within 12. Travelers are good-to-go after downloading and printing the report. For a premium, results can be returned within five hours.
“This new RT-PCR has the potential to be a gamechanger in the fight against COVID-19, not just for Saudi Arabia but also for other countries looking for economical, easily locally administered tests,” KAUST President Tony Chan said. “This is also a powerful example of a KAUST startup, NoorDX, collaborating with a breakthrough technology developed by faculty at KAUST and bringing a commercially-ready technology out of the lab to benefit Saudi society.”
In addition to being the first Saudi test kit provider, Noor DX will be the first genomics entity in the Kingdom with fully localized capability, offering a portfolio of genomic services beginning in early 2022. Currently, the majority of clinical tests are sent abroadand localized production is strategic to building genomics expertise within the Kingdom.

The healthcare spinout has previously received seed investment from KAUST Innovation Ventures, the University’s venture capital arm, and has also been supported in this initiative by the KAUST Core Labs and Research Infrastructure, and Department of Health, Safety and Environment to build out the laboratory facility which will be operated by the startup.
Noor DX CEO Dr. Abdulaleh Alhawsawi said, “This is a great moment in time, both for KAUST’s ability to provide quality RT–PCR tests, and soon, gene sequencing. Noor DX will cater to Saudi communities with a full range of services and downstream applications, from dry to wet lab technologies to analytics to building the Saudi biobank and national genome.”

KAUST is transforming patient care through its partnerships. Noor DX is among a constellation of experts contributing to robust health services from the areas of academia, economic innovation, biotechnology and biopharma, including Dr. Pierre Magistretti, director of the Smart-Health Initiative; Dr. Samir Hamdan and the Rapid Research Response team; Dr. Arnab Pain, director of the Pathogen Genomics Laboratory; KAUST Innovation; the Core Labs; Office of the President; Office of Research; Department of Health, Safety and Environment; and KAUST Health.