New Pincer Type Ligand Catalysts For Green Industrial Uses
Cleaner, greener chemical reactions ready for organic synthesis and industry
Ethyl acetate is a compound with industrial significance used either as a solvent or a reaction intermediate.
Current means of producing ethyl acetate, however, rely on processes such as the esterification of acetic acid with ethanol, a procedure that requires the use of a petrochemical and expensive additional distillation steps to arrive at a solvent of the desired purity for industrial applications.
Researchers at KAUST are working on ways to make this esterification process greener and more efficient. That is how a new class of pincertype ligands has been discovered.
This work has earned the first KAUST issued patent and is poised to move into industrial application.
Technology Summary
The subject technology is a new class of ligands and their corresponding ruthenium catalysts capable of catalyzing a variety of dehydrogenation reactions.
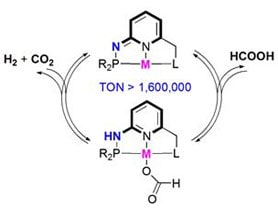
How It Works
The pincer complexes are based on different metals that are suitable for different applications, including ethanol to ethyl acetate, selective hydrogen generation from formic acid, ethylene polymerization, 1,3butadiene polymerization to 1,4cispolybutadiene, etc. These reactions are very efficient, converting nearly all of the initial substrates into the desired products suitable for industrial use.

Why It Is Better
In the case of the production of ethyl acetate, this new class of pincer ligand when complexed with various metals it allows direct conversion of ethanol through a “dehydrogenative coupling” process. The reaction utilizes ethanol, which can be a biorenewable compound, and as a result, provides more sustainable reaction that does not rely on petrochemicals.
Another important application of pincer ligands catalysts is using them to selectively dehydrogenate formic acid, a novel method of hydrogen storage. This has long been a goal for the sustainable energy movement, because the problem of storage and transport of hydrogen has been a major issue stalling the broader applications of hydrogen as a fuel. For instance, if the fuel cell system could carry formic acid in liquid form and then use a chemical reaction catalyzed by a pincer complex to release hydrogen fuel when needed, the resulting cells would be a safer and more efficient system for powering electric cars.
IP Protection
KAUST has an issued patent 8,598,351 for this technology. KAUST has the exclusive rights on this technology and the know-how in collaboration with University of Toronto.
Invention Track Code
2011-041

Benefits
- Flexibility: variation in the catalyst structure, diversity of reactivity and application
- High performance: Increases resonant frequency to at least 20 GHz, which allows for use in higher bandwidth applications
- Clean and Green Reactions:
- Neat substrate reactivity
- Less waste
- Recyclable

Applications
The new class of pincer complexes provides new opportunities in organic synthesis and process for industries such as:
- Pharmaceutical
- Cosmetic
- Agrochemical
- Food
- Petrochemical
